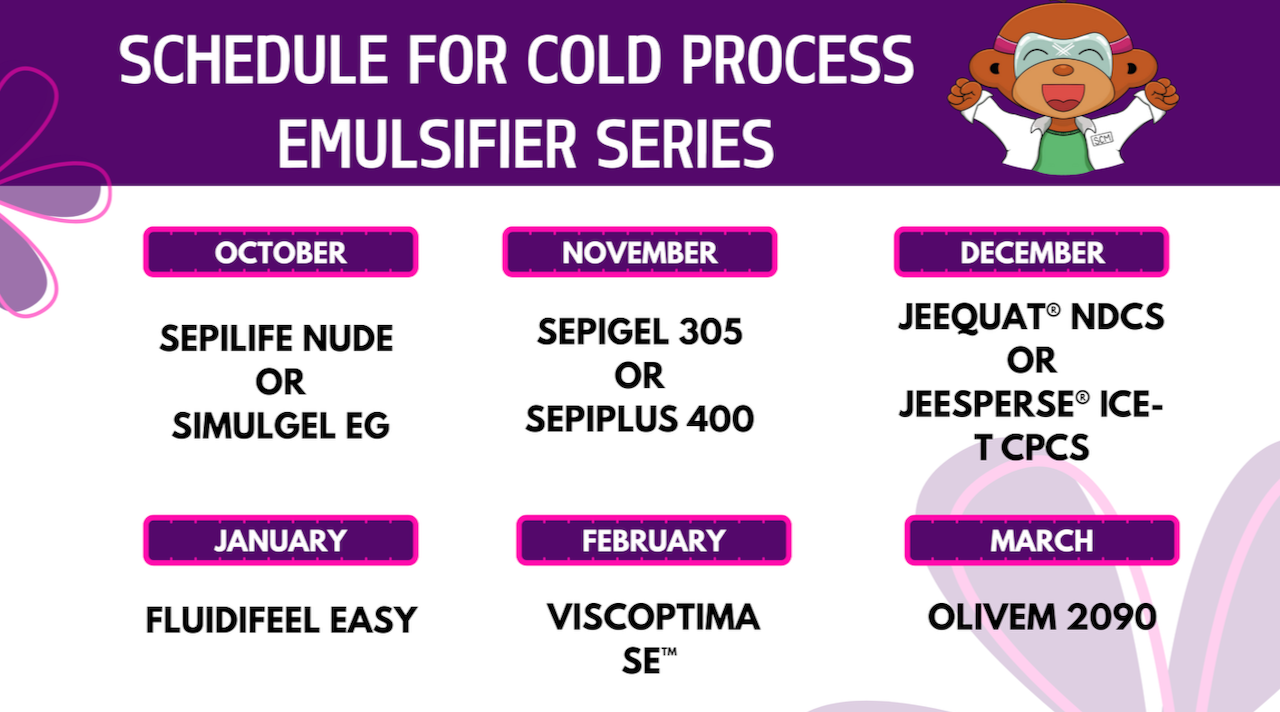
Quick reminder: Don’t forget about the upcoming cold process emulsifier series!
Woo hoo! We’re starting our new introduction to cold process emulsifiers series in October 2025 that’ll be available to Foundation, Formulation, and Innovation subscribers to the site here. Starting October 3rd, 2025, every Friday – for Formulating Friday – we’ll be playing with awesome cold process emulsifiers that’ll help us make emulsions in 10 minutes...