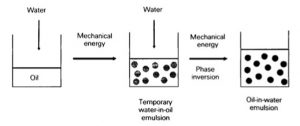
 How exactly does phase inversion work?
How exactly does phase inversion work?
Most of the emulsifiers we use are ethoxylated fatty materials. The hydrogen bonding between the ethoxylated molecules and the water is what helps with the emulsion.
When they are heated two things happen: their HLB changes from about 12 to about 5, which means they are more suitable for water-in-oil emulsions, and their ability to form these hydrogen bonds with water decreases. So they’re really acting as water-in-oil emulsifiers when heated.
As the lotion cools, the HLB of these emulsifiers starts to increase back to the proper HLB value and their ability to hydrogen bond with water increases. The lotion switches from being a water-in-oil emulsion to being an oil-in-water emulsion.
This is more stable because the oil droplets in a phase inverted lotion will be smaller than those found in a non-phase inverted lotion.
We know that oil droplet clumping can be one of the biggest reasons for separation – the smaller the droplets, the easier it is to keep them apart, thus less likelihood of clumping.
Smaller, sturdier micelles are less likely to collide, but when they do, they will remain as the small micelle instead of coalescing into a larger one or spilling out the oils into the water.
