Why are we adding some ingredients to the cool down phase? Why do my emulsions lose viscosity when I add some preservatives? How do I make it not lose viscosity? (Part two)
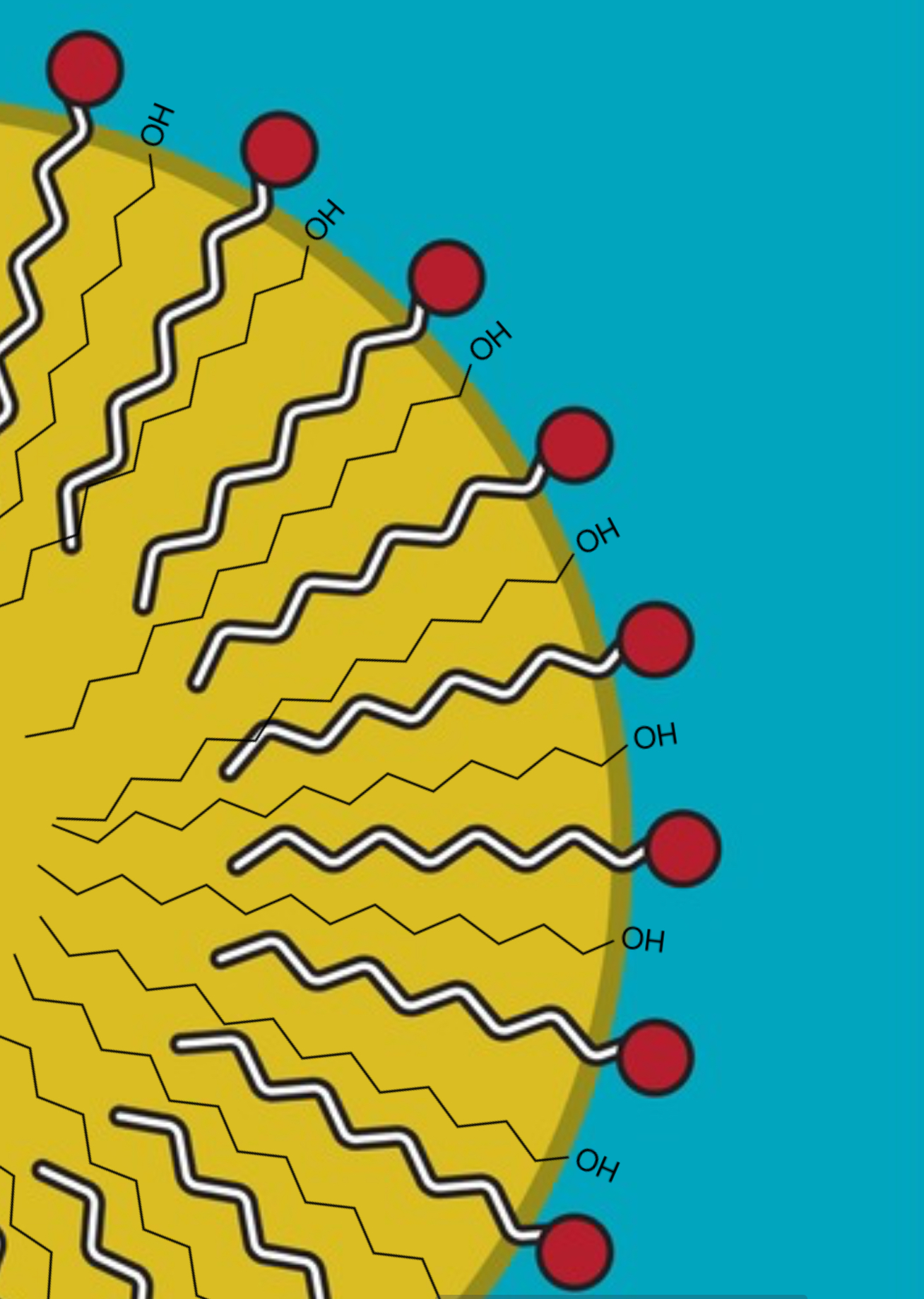
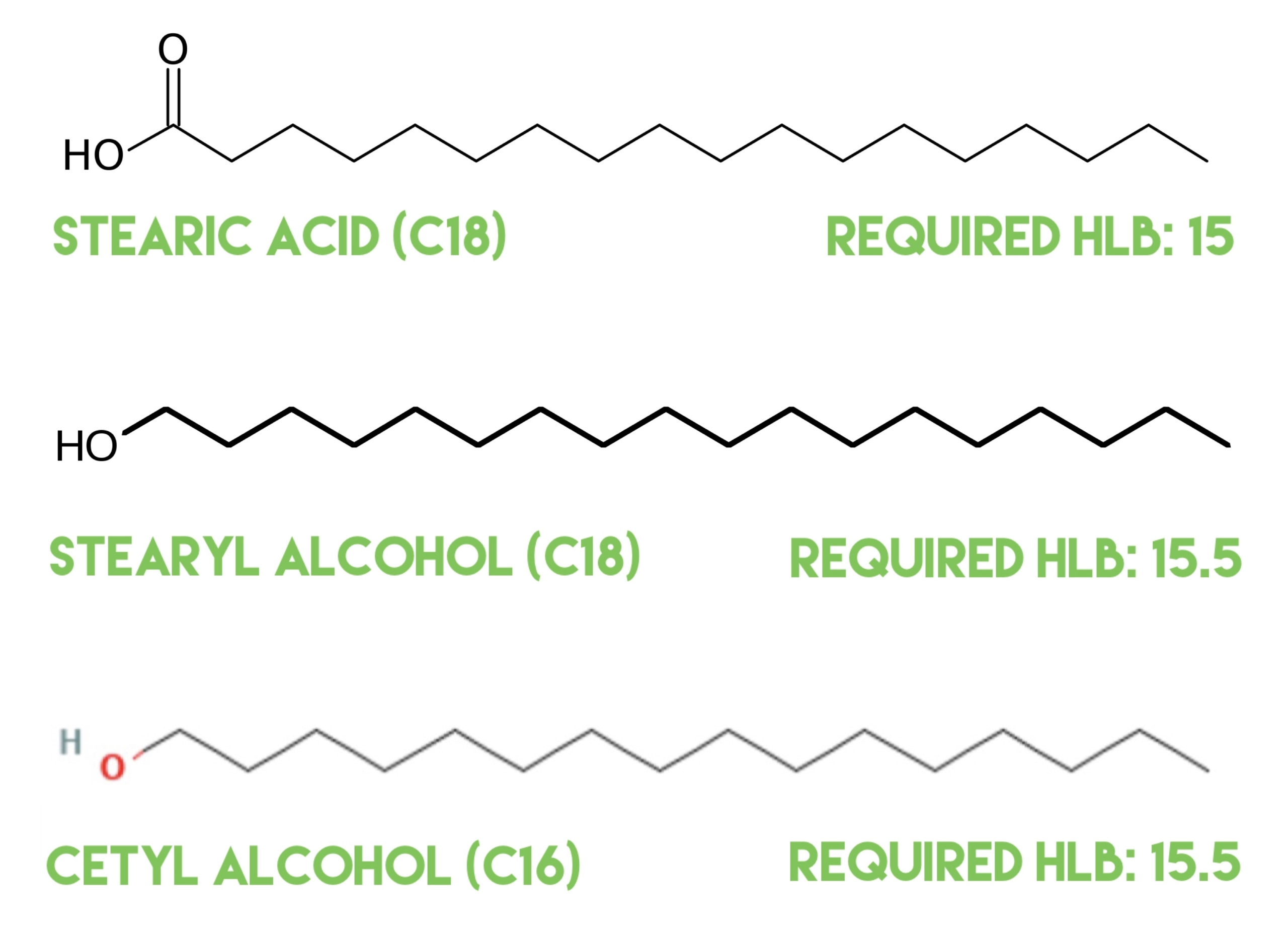
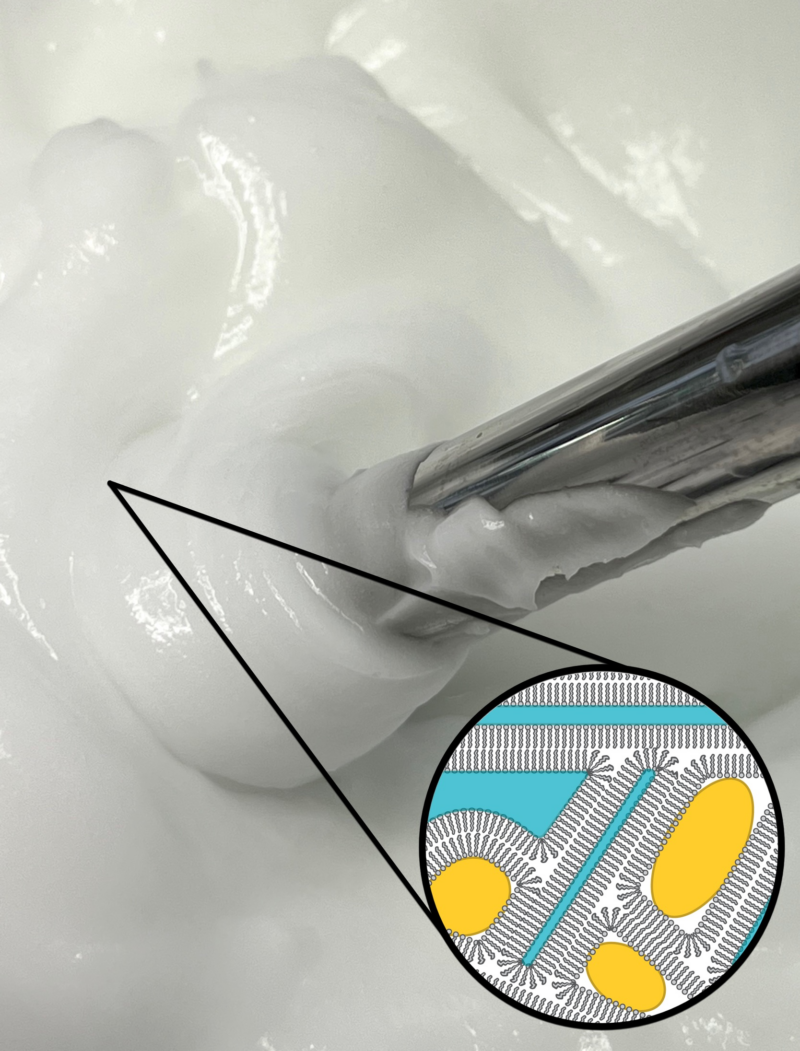
Welcome back to part of two of this short series in which we’re looking at why we might add some ingredients to the cool down phase of a formula. In part one, we looked at why we added hydrolyzed proteins, mixed tocopherols, actives & cosmeceuticals, fragrance oils, and essential oils to the cool down phase...