A liquid crystal emulsifier is one that helps to create oil-in-water liquid crystal emulsions, which is a bilayered structure rather than just the round micelles we normally think of when we talk about emulsions. These are called lamellar gel networks.
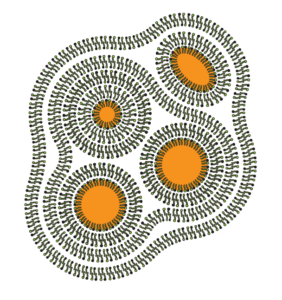
This is what a lamellar gel network might look like. The yellow bits are the oil, the black spiky bits are emulsifier, and the white part is water. You can see that we have oil in the micelles with water around them, but there’s more emulsifier with more water around those micelles. That’s the lamellar gel network. It isn’t just as simple as having some bubbles/micelles floating in water.
They can hold more water in the structure, trapped between the layers, which can deliver more water and hold it longer on our skin. This may decrease transepidermal water loss, increase hydration, cause less disruption to our stratum corner, and may deliver actives such as cosmeceuticals and vitamins to our skin better. They can be more stable than those with spherical micelles.
The key is to combine these emulsifiers with structuring agents – fatty alcohols, fatty acids, glyceryl stearate, solid esters – to create more sturdy structures like those in the above diagram. So when we’re using these types of emulsifiers, if they don’t have a structuring agent, we’ll want to add one.
Learn more about fatty alcohols – cetearyl alcohol, cetyl alcohol, behenyl alcohol, and other alcohols – as well as other structuring agents in the fatty alcohol section of the emollients section of the blog.
There are other properties to consider – melting point of the ingredients, the hardness of the rings that are created, hydrogen bonding with the water in the rings, the proportion of emulsifier to structuring agent – but we’ll get into that in the extended series.
